Researcher's progress makes artistic journal cover story
Tuesday, April 1, 2014
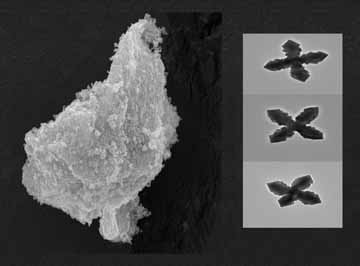
“We made materials that resemble German crosses and what we call a ‘micro chicken’
by varying the concentrations of reactants we use in a new process to produce nanocrystals
of a mineral called lead molybdate,” said Apblett. “The same process can be fine-tuned
for extensive applications and uses in optical, electronic and sensor devices and
as catalysts for chemical reactions used in the preparation of petrochemicals, pharmaceuticals
and water purification.”
The nanocrystal building blocks, which resulted in the unusual magazine cover designs,
are more than 2,000 times smaller than the diameter of a human hair, according to
Apblett. They were photographed as black and white images called electron micrographs
using OSU's electron microscopes.
Nanocrystals and nanoparticles are important because they allow scientists to do
things not possible with the larger particles, explained Apblett. “For instance,
gold nanoparticles are often red in color and not gold like their larger counterparts.
This color difference shows that the electronic properties have changed, making gold
nanoparticles useful sensing materials and as catalysts. Unfortunately, lead molybdate
doesn’t show a remarkable color change, but in its nanocrystalline form, it does display
extremely useful catalytic, optical, and electronic properties.”
Apblett, professor of chemistry in the College of Arts and Sciences at OSU, is working
with graduate student Cory Perkins on this process that was also investigated by OSU
alumnus Dr. Kevin Barber. Their research article appears in the April 14, 2014 edition
of CrystEngComm, which is published by the Royal Society of Chemistry, based in Great
Britain.
